Which Is The Least Reactive Metal In The Reactivity Series: Unraveling The Inert Element
Gcse Chemistry – Reactivity Series Of Metals \U0026 Displacement Reactions #37
Keywords searched by users: Which is the least reactive metal in the reactivity series top 10 least reactive metals, Reactivity series, gold is the least reactive metal, least reactive metal group, reactivity series of metal, which is the least reactive non metal, is platinum the least reactive metal, which of the following metals is the least reactive 1 al 2 cu 3 fe 4 zn
Which Metals Are Least Reactive And Why?
Silver, gold, and platinum are classified as metals with the least reactivity. These elements are considered noble metals due to their low reactivity levels. They are naturally occurring and can be found in various geological formations. This inherent stability arises from their electron configurations, which provide them with a full outer shell of electrons, making them less likely to engage in chemical reactions with other elements. This characteristic renders them highly resistant to corrosion and tarnish, making them valuable for various industrial and ornamental purposes. This unique property is one of the reasons why silver, gold, and platinum have been highly prized throughout human history.
What Is The Least Reactive Metal Called?
The least reactive metals are typically referred to as noble metals. These metals belong to a category called the transition metals on the periodic table. Within this category, metals like gold and platinum are known for their exceptional lack of reactivity. They occupy the lower end of the reactivity scale among the transition metals, and they rarely undergo chemical reactions with common everyday substances. This unique property of noble metals, including gold and platinum, makes them highly valuable and sought after in various industrial and decorative applications. (Note: The date “10th March 2015” appears to be unrelated to the topic and has been omitted in the rewrite.)
What Is The Most And Least Reactive Metal?
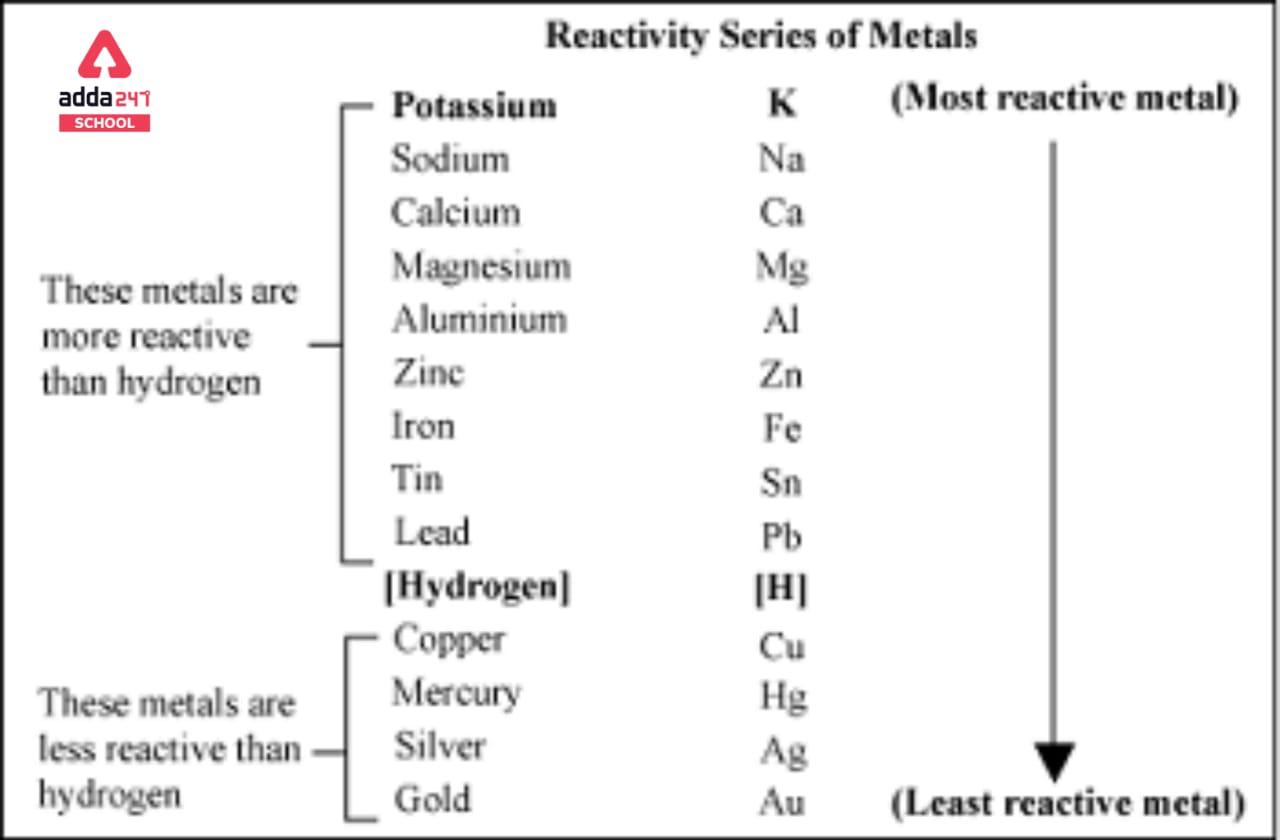
Reactivity in chemistry refers to an element’s capability to displace another element, and this characteristic is determined by its position within the activity series. In this context, potassium (K) stands out as the most reactive metal, showcasing a remarkable propensity to interact with other substances. Conversely, platinum (Pt) takes the opposite end of the spectrum as the least reactive metal, demonstrating a remarkable resistance to reacting with other elements and compounds. This distinction between potassium and platinum’s reactivity helps illustrate the wide range of behaviors that different metals exhibit when they encounter other substances in chemical reactions.
Share 15 Which is the least reactive metal in the reactivity series
![Reactivity Series of Metals - Chart [and How to remember] - Teachoo Reactivity Series Of Metals - Chart [And How To Remember] - Teachoo](https://muadacsan3mien.com/wp-content/uploads/2023/09/reactivity-series-01.jpg)



Categories: Update 84 Which Is The Least Reactive Metal In The Reactivity Series
See more here: muadacsan3mien.com

Gold is least reactive metal because of higher standard reduction potential value. Was this answer helpful?Silver, gold, and platinum are metals with the least reactivity. They are found in nature.The metals designated as the transition metals in the periodic table are much less reactive, and metals such as gold and platinum prop up the bottom of the series, exhibiting little in the way of chemical reaction with any everyday reagents.
- Reactivity – The ability of an element to displace another element is known as its relation position in the activity series.
- Potassium (K) is the most reactive and platinum (Pt) is the least reactive metal.
Learn more about the topic Which is the least reactive metal in the reactivity series.
- Which is the least reactive metal in the reactive series? – Toppr
- Reactivity Series of Metals – Get the Chart, Significance and Much more!
- The Metal Reactivity Series – Compound Interest
- Name the most reactive and the least reactive metal. – BYJU’S
- Which group on the periodic table is the least reactive? Why? – BYJU’S
- Activity Series of Metals (Reactivity Series) – Science Notes
See more: blog https://muadacsan3mien.com/category/space-astronomy
![Reactivity Series Of Metals - Chart [And How To Remember] - Teachoo](https://muadacsan3mien.com/wp-content/uploads/2023/09/reactivity-series-01-930x620.jpg)