What Are Exothermic And Endothermic Changes? Examples Unveiled!
What Are Endothermic \U0026 Exothermic Reactions | Chemistry | Fuseschool
Keywords searched by users: What are exothermic changes and endothermic changes give one example for each name one type of change that can be exothermic or endothermic, example of endothermic change, give an example of exothermic change, define exothermic and endothermic changes give two examples in each case, two example of endothermic change, write two reactions each showing exothermic and endothermic nature., what are exothermic change give two examples, what is endothermic change
What Is Exothermic Change With Example?
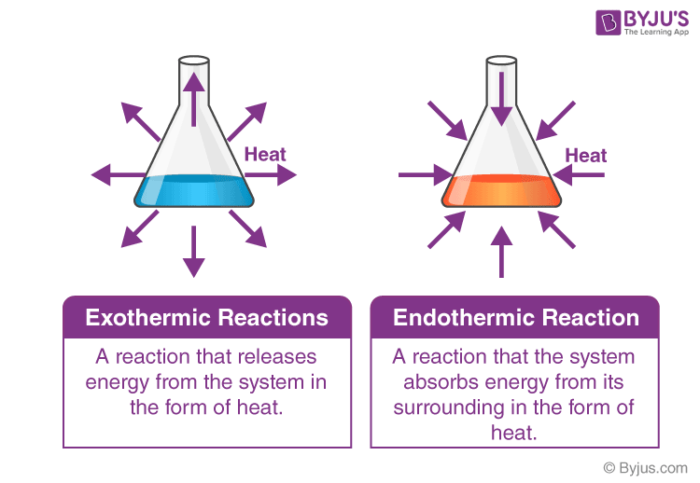
An exothermic change refers to a chemical or physical process that releases heat energy into the surrounding environment. Let’s explore this concept with a specific example involving the burning of a candle.
When you light a candle, it consists of a wick surrounded by paraffin wax, which is a hydrocarbon. As the candle burns, a chemical reaction takes place between the paraffin wax and oxygen in the air. This reaction generates two primary products: carbon dioxide (CO2) and water (H2O). Importantly, this process is classified as exothermic because it releases a significant amount of heat energy into the surroundings.
So, in summary, the act of burning a candle is an example of an exothermic change because it involves the exothermic combustion of paraffin wax, resulting in the production of heat, carbon dioxide, and water.
What Is Endothermic Change With Example?
What is an endothermic change, and can you provide an example? Endothermic changes are processes that absorb heat from their surroundings, causing a decrease in the temperature of their immediate environment. An illustrative example of an endothermic change is the melting of ice. When ice cubes undergo this process, they absorb heat energy from their surroundings, causing them to transition from a solid state to a liquid state. This absorption of heat is what makes the surrounding area feel colder during the melting process, as the ice “takes in” heat to facilitate the transformation.
Aggregate 46 What are exothermic changes and endothermic changes give one example for each

:max_bytes(150000):strip_icc()/endothermic-reaction-examples-608179_FINAL-5d1c7be66fdb48878ae5842f4a873da6.png)


Categories: Collect 98 What Are Exothermic Changes And Endothermic Changes Give One Example For Each
See more here: muadacsan3mien.com

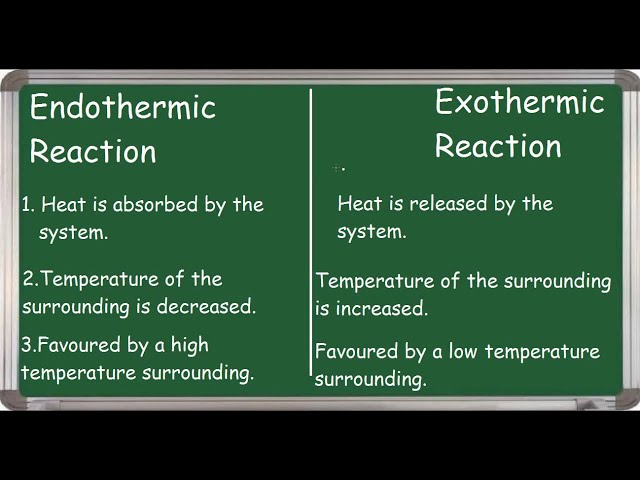
In the case of burning a candle, the body of the candle carries paraffin, which is a hydrocarbon. When paraffin burns with the flame, it reacts with oxygen to create carbon dioxide and water. This reaction is certainly exothermic as it gives out heat to the atmosphere with the help of other products.Example – Melting of ice is an endothermic change in which ice cubes are melted by heat absorbed from the surrounding.exothermic reaction is in which heat is released and endothermic reaction is in which heat is absorbed. exothermic reaction – Combustion of fuels such as wood, coal and oil petroleum. Condensation of rain from water vapours. endothermic reaction – Melting ice cubes.
| Exothermic processes | Endothermic processes |
|---|---|
| rusting iron | cooking an egg |
| burning sugar | producing sugar by photosynthesis |
| forming ion pairs | separating ion pairs |
| Combining atoms to make a molecule in the gas phase | splitting a gas molecule apart |
Learn more about the topic What are exothermic changes and endothermic changes give one example for each.
- Define exothermic and endothermic changes. Give two …
- IIT JEE Chemistry Exothermic Reactions Real Life Examples
- Define endothermic change with an example. – BYJU’S
- define exothermic and endothermic changes give two …
- List the types of physical changes that can yield exothermic and … – Vaia
- Effect of Change of Temperature – GeeksforGeeks
See more: blog https://muadacsan3mien.com/category/space-astronomy